
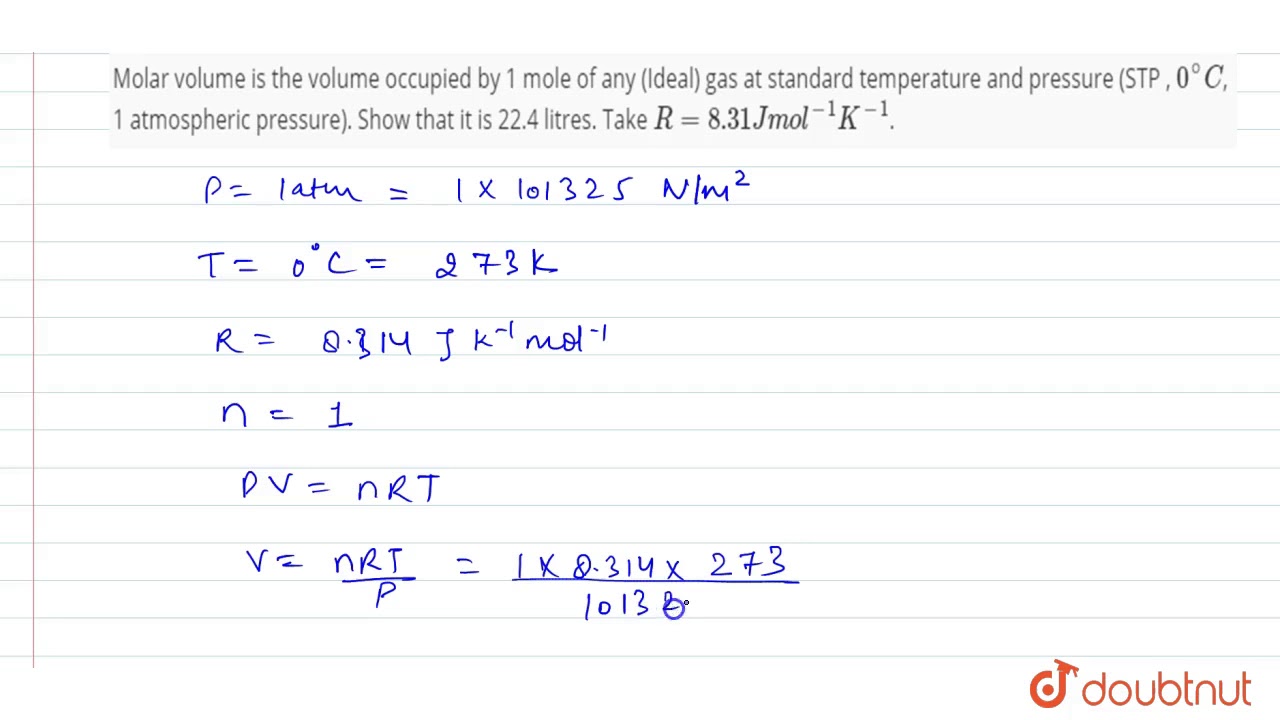
R = d / (2 2) <- one of two alternate formulationsģ) Determine the volume of the unit cell: Use the Pythagorean Theorem (see problem #1 for a discussion): Assuming that calcium has an atomic radius of 197 pm, calculate the density of solid calcium.ġ97 pm x (1 cm/10 10 pm) = 1.97 x 10¯ 8 cmĢ) Determine the edge length of the unit cell: Problem #4: Calcium has a cubic closest packed structure as a solid. (See problem 5a below for an example set of calculations.)ģ) Calculate the average mass of one atom of NiĤ) Calculate the mass of the 4 nickel atoms in the face-centered cubic unit cellĥ) Calculate the density (value from step 4 divided by value from step 2) This problem is the exact reverse of problem #2. Problem #3: Nickel has a face-centered cubic structure with an edge length of 352.4 picometers. This problem is like the one above, it just stops short of determining the atomic radius.ġ) Calculate the average mass of one atom of Ni:ĥ8.6934 g mol¯ 1 / 6.022 x 10 23 atoms mol¯ 1 = 9.746496 x 10¯ 23 g/atomĢ) Calculate the mass of the 4 nickel atoms in the face-centered cubic unit cell: If the density of the metal is 8.908 g/cm 3, what is the unit cell edge length in pm? Problem #2: Nickel crystallizes in a face-centered cubic lattice. You may be asked to do the opposite, that is, to determine d in terms of r for a fcc cell. The above discusses how to determine r in terms of d in a face-centered unit cell.

Try it before looking at the solution to the next problem. You may wish to convert the cm value to picometers, the most common measurement used in reporting atomic radii. R = d / (2 2) <- an alternate formulation Here is the same view, with 'd' representing the side of the cube and '4r' representing the 4 atomic radii across the face diagonal. The square represents one face of a face-centered cube: Remember that a face-centered unit cell has an atom in the middle of each face of the cube. Calculate the atomic radius of palladium.ġ) Calculate the average mass of one atom of Pd:ġ06.42 g mol¯ 1 / 6.022 x 10 23 atoms mol¯ 1 = 1.767187 x 10¯ 22 g/atomĢ) Calculate the mass of the 4 palladium atoms in the face-centered cubic unit cell: Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Face-centered cubic problems Face-centered cubic problems


 0 kommentar(er)
0 kommentar(er)
